COVID-19 vaccination guidelines: boosters, codes, indications, and timing
THOMAS WEIDA, MD, FAAFP, AND JANE WEIDA, MD, FAAFP
On Nov. 2, the director of the Centers for Disease Control and Prevention (CDC) gave the final OK on Pfizer’s low-dose COVID vaccine for children ages 5 to 11, following the Oct. 29 emergency use authorization by the Food and Drug Administration (FDA). In late October, the FDA and CDC also expanded the availability of COVID-19 booster vaccines in eligible populations and approved the use of any of the three available vaccines to booster any primary series (“mix and match” dosing). The primary series should be given with the same vaccine and not interchanged with another vaccine.
To keep up with the changing requirements related to COVID-19 vaccines — including boosters, coding, and vaccines for kids — practice staff can use the following tables as a quick reference guide.
The COVID-19 vaccines available in the U.S. have different age ranges, doses, and dosage schedules, as well as different product CPT codes and different administration codes depending on the dose given. Table 1 provides a summary of the coding for each vaccine. When coding, indicate the product code and the administration code on the claim form.
Table 1: COVID-19 vaccine scheduling and coding guidelines
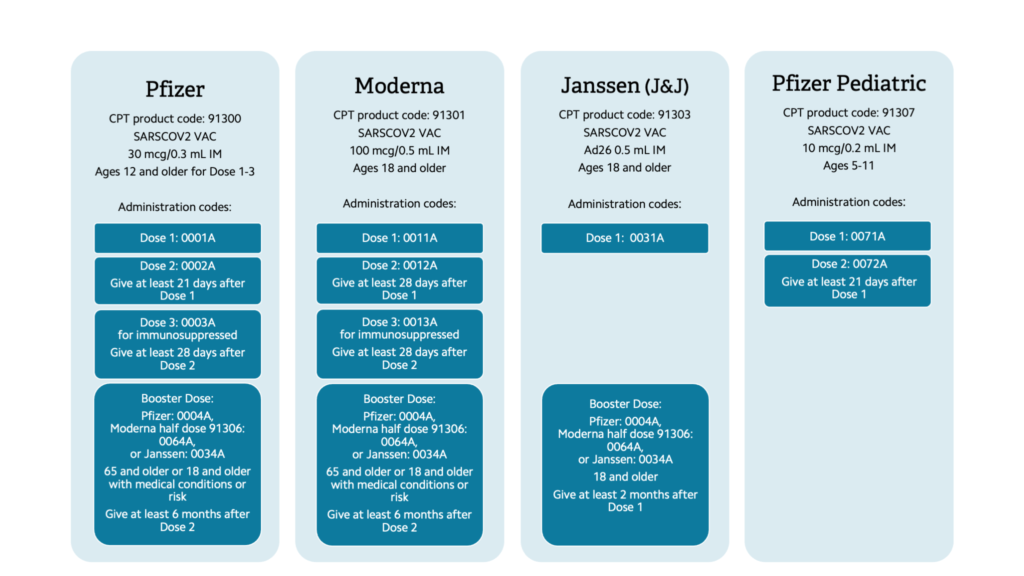
The Pfizer vaccine is now marketed under the brand name COMIRNATY but has the same formulation and indications as the EUA Pfizer vaccine. Pfizer also has a ready-to-use COVID-19 vaccine that does not need to be reconstituted. The product code is 91305. The first dose administration is coded as 0051A, the second as 0052A, the third as 0053A, and the booster as 0054A.
Third dose indications
A third dose of COVID-19 vaccine is given when a two-dose primary series is deemed insufficient, such as in individuals who are moderately to severely immunocompromised. Indications for the third dose of the Pfizer or Moderna vaccine, which are given at least 28 days after the second dose, are in Table 2.
Table 2: Indications for third-dose COVID-19 vaccine
| Receiving active cancer treatment for tumors or cancers of the blood |
| Organ transplant recipient taking immune suppression medication |
| Stem cell transplant within last two years |
| Moderate or severe primary immunodeficiency (e.g., Wiskott-Aldrich) |
| Advanced or untreated HIV infection |
Booster dose indications
A booster dose is given when the initial primary COVID-19 vaccine series was sufficient but has decreased over time. In the case of Pfizer or Moderna vaccines, the booster dose is given at least six months after the primary series. In the case of Janssen vaccine, the booster dose is given at least two months after the first dose. Indications for the COVID-19 booster are in Table 3. The Moderna booster vaccine is half the dose of the initial vaccine, and no more than 20 doses should be given from a 10 mL vial.
Table 3: COVID-19 booster shot indications
| Co-morbidities supported by meta-analysis/systematic review | Co-morbidities supported by observational studies | Co-morbidities supported by case series, case reports, or small sample size | Co-morbidities supported by mixed evidence |
|---|---|---|---|
| Cancer Cerebrovascular disease Chronic kidney disease* COPD (chronic obstructive pulmonary disease) Diabetes mellitus, type 1 and type 2* Heart conditions (such as heart failure, coronary artery disease, or cardiomyopathies) Obesity (BMI ≥30 kg/m2)* Pregnancy and recent pregnancy Smoking, current and former | Down syndrome HIV (human immunodeficiency virus) Neurologic conditions, including dementia Overweight (BMI ≥25 kg/m2, but <30 kg/m2) Other lung disease (including interstitial lung disease, pulmonary fibrosis, pulmonary hypertension* Sickle cell disease Solid organ or blood stem cell transplantation Substance use disorders Use of corticosteroids or other immunosuppressive medications | Cystic fibrosis Thalassemia | Asthma Hypertension Immune deficiencies Liver disease |
Payments and cost sharing
Medicare payment allowances and effective dates are available from the Centers for Medicare & Medicaid Services (CMS). The agency recently reminded eligible patients that coverage for the booster shots with no cost sharing is available under Medicare, Medicaid, and the Children’s Health Insurance Program, as well as in the commercial health insurance market.
Additional resources:
- AAFP Livestream: Addressing the Use of COVID-19 Vaccines in Children,
- AAFP COVID-19 Booster Doses FAQ,
- CMS COVID-19 provider toolkit, which addresses payment rates for administering vaccines and how to bill correctly.
— Thomas Weida, MD, FAAFP, is Chief Medical Officer, Associate Dean for Clinical Affairs, Professor, The University of Alabama College of Community Health Sciences. Jane Weida, MD, FAAFP, is Chair, Department of Family, Internal and Rural Medicine, Professor, The University of Alabama College of Community Health Sciences.
SOURCES:
Table 1:
https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/covid-19-vaccines-and-monoclonal-antibodies
https://www.cdc.gov/media/releases/2021/p0924-booster-recommendations-.html
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-10-20-21/12-COVID-Reddy-508.pdf
https://www.fda.gov/media/144413/download
https://www.ama-assn.org/practice-management/cpt/covid-19-cpt-vaccine-and-immunization-codes
Table 2:
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html
